lifestyle
How Long Does Compounded Semaglutide Last in the Fridge?

If you’re using compounded semaglutide, proper storage is essential to ensure it remains effective and safe. But how long can it actually last in the fridge before it loses potency? Understanding the shelf life of compounded semaglutide helps you plan doses, reduce waste, and maximize the benefits of your treatment. In this guide, we break down the facts, best storage practices, and tips to make sure your medication works exactly as intended.
To put it simply, MeAgain’s GLP-1 app helps you track refrigerated storage, set expiry reminders, and record fridge temperature so you stay on top of vial and pen stability without extra stress.
Summary
Compounded semaglutide does not have a universal refrigerated shelf life; most vials and prefilled syringes are expected to remain viable for 30 to 90 days, so the pharmacy-assigned beyond-use date should govern use.
Analytical testing and reporting show batch-to-batch potency can vary by up to 20%, and many compounding pharmacies set conservative BUDs of 30 to 60 days when they lack extensive in-house stability data.
Proper storage matters: store compounded semaglutide at 2°C to 8°C (36°F to 46°F) in the main refrigerator compartment, not on the door, because freezing or temperature excursions accelerate degradation.
Some preparations should be used within 30 days of opening. While brief room-temperature handling for a few minutes is generally tolerated, exposures measured in hours should prompt a call to the dispensing pharmacy.
Although a few sources report stability claims of up to 6 months under ideal test conditions, such longer timelines depend on validated real-time stability studies and specific container-closure systems, rather than on universal practice.
GLP-1 app addresses this by centralizing beyond-use dates, batch numbers, temperature logs, and expiry reminders so storage events and risks are documented for users and clinicians.
Table of Content
Why Compounded Semaglutide Has a Shorter, Variable Shelf Life
How to Store Compounded Semaglutide Safely and When to Stop Using It
How Long Does Compounded Semaglutide Last in the Fridge?
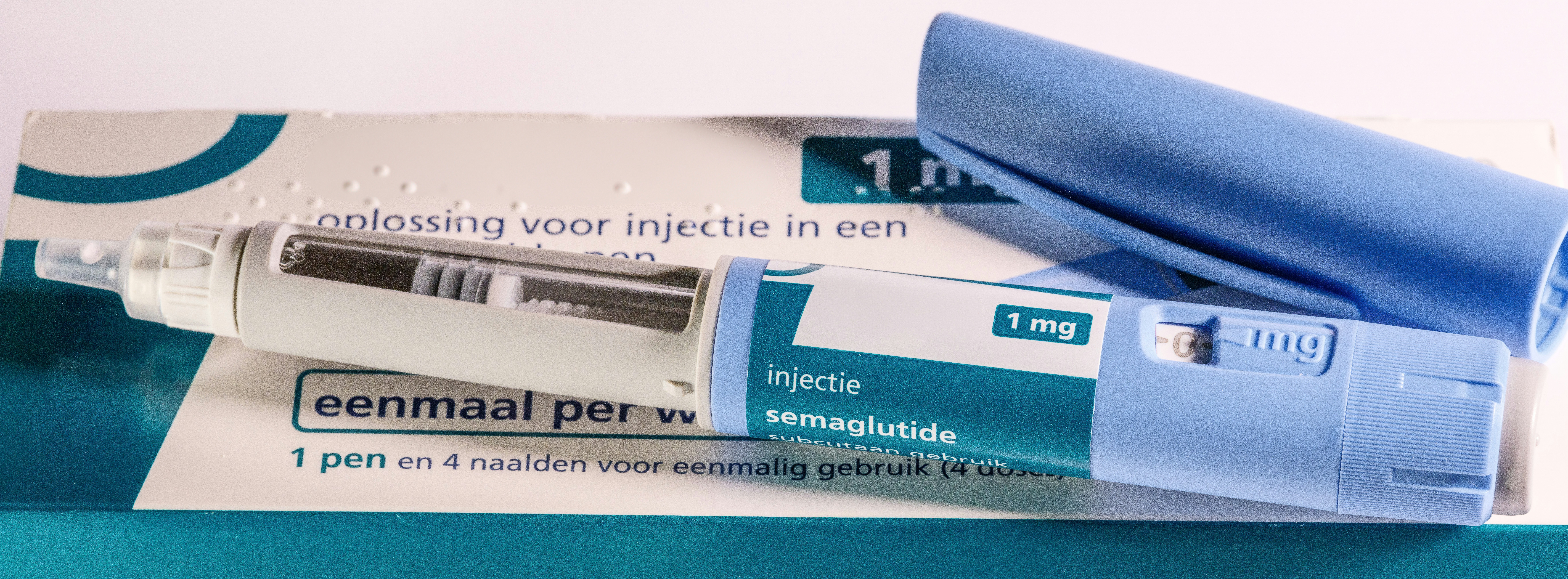
No universal fridge lifespan: Most compounded semaglutide vials or prefilled syringes have a shelf life of 30–90 days, depending on the pharmacy-assigned beyond-use date (BUD). Always follow your pharmacy’s instructions.
Batch potency varies: Testing shows up to 20% variability between batches; conservative BUDs (30–60 days) are common when stability data is limited.
Ideal fridge conditions: Store in the main compartment at 2–8°C (36–46°F), not in the door. Avoid freezing or temperature fluctuations.
Opened vials have shorter use windows: Some should be used within 30 days of opening to reduce the risk of contamination.
Room-temperature handling: Short exposure (a few minutes) is acceptable, but hours at room temperature may require consulting your pharmacy.
Tracking helps: Using tools like MeAgain to log BUDs, fridge temperature, and handling events reduces waste and ensures consistent dosing.
Compounded semaglutide does not have a single, universal fridge lifespan; it depends on the beyond-use date set by the compounding pharmacy and how the product was prepared. In practice, most compounded vials and prefilled syringes are expected to remain viable in the refrigerator for roughly 30 to 90 days, but you must follow the pharmacy’s BUD and discard anything past that date.
Why Does Compounded Semaglutide Need To Be Refrigerated?
Compounded semaglutide is a peptide, and peptides lose structure when exposed to fluctuating or extreme temperatures, which reduces potency and can change safety profiles.
Refrigeration stabilizes the molecule by:
Maintaining a stable temperature
Slowing chemical degradation
Reducing the risk of contamination during repeated vial access
Improper storage can mean weaker blood sugar control or wasted medication, which is exactly why people feel anxious when the expiration window is unclear.
How Long Can Compounded Semaglutide Stay In The Fridge?
The recommended refrigerator duration for compounded semaglutide depends on the product's beyond-use date (BUD) and varies by compounding pharmacy. In most cases, compounded semaglutide can be stored in the refrigerator for 30-90 days.
After that, it may not be effective or safe to use. Be sure to check the expiration date and shelf life printed on your prescription; discard expired or unused medication as directed by your healthcare provider or pharmacist.
What About Manufacturer Guidance Or Reported Stability Limits?
Some compounding sources report longer stability under ideal conditions, and that influences how some pharmacies set BUDs; according to ShapeIn Rx, compounded semaglutide can last up to 6 months in the fridge, a claim tied to specific storage and testing assumptions rather than universal practice.
Use that kind of claim only as a conversation starter with your pharmacist, because the BUD you receive is the legally supported timeline you should follow.
How Long Can Compounded Semaglutide Stay Out Of The Fridge?
Brief handling at room temperature while you draw and inject, for a few minutes, will not ruin a properly stored vial. If a vial or prefilled syringe was accidentally left at room temperature for hours, you should call the dispensing pharmacy and describe the timing and exposure, because they will tell you whether to discard it.
That step matters because subtle, time-dependent degradation is difficult to detect by eye.
Should I Use Semaglutide After Opening A Vial?
Some preparations include short-use instructions once opened, reflecting contamination risk from needle access; in fact, ShapeIn Rx notes that Semaglutide should be used within 30 days of opening, a practical limit tied to sterility and predictable potency.
Follow the pharmacy’s explicit instructions for opened vials, as an opened but older vial has a different safety profile than an unopened one.
Can I Use Compounded Semaglutide Past Its Beyond-Use Date?
No, you should not. The BUD is the pharmacist’s assessment of when potency and sterility can no longer be guaranteed. Using medication past its BUD risks subpotent treatment and unpredictable effects, which means lost progress and possible health risk.
The ‘Sunk Cost’ Trap: Balancing Cost vs. Clinical Safety
This is stressful because the system that:
Creates variability
Compounding pharmacies
Patient behavior interacts badly
The common approach is to treat a refrigerated vial as “good until it runs out,” because it feels wasteful to discard expensive medication. That logic breaks down when BUDs vary, and storage logs are informal, producing confusion and anxiety about wasted doses and missed outcomes.
From Mental Notes to Digital Accuracy: Managing Multi-Vial Complexity
Most people manage storage and dosing with mental notes or paper logs. That works early on, but when vials, prefilled syringes, and refills come from different pharmacies, tracking BUDs and room-temperature exposures becomes error-prone and costly.
Platforms like MeAgain provide:
Dosing reminders
Centralized logs for batch numbers and BUDs
Alerts when a vial is approaching its beyond-use date
It helps users avoid accidentally using expired products and reduces unnecessary waste.
The Safety-Cost Conflict: Breaking the Cycle of Storage Anxiety
A pattern we see repeatedly across clinics and home users is this:
Inconsistent BUD communication
Combined with anxiety about cost
leads many to push the limits of storage
It creates a failure mode where people either toss medication prematurely out of fear or use it too long out of convenience, and both outcomes hurt results and peace of mind.
If you want practical next steps, I use with patients:
Always record the BUD on the vial the moment you get it
Note each time a vial leaves the fridge and for how long
When in doubt, call your compounding pharmacy with exact timing
Those simple, documented actions remove most guesswork and prevent costly errors before they occur. That apparent clarity raises a sharper question: why do some compounded products have shorter, inconsistent shelf lives, and why do those differences matter so much?
Related Reading
Why Compounded Semaglutide Has a Shorter, Variable Shelf Life

There is no single FDA-approved fridge lifespan for compounded semaglutide; the usable window is whatever the compounding pharmacy can justify based on testing and quality controls, and refrigeration only slows chemical and microbial degradation; it does not stop either.
To manage these tight windows effectively, many patients use the MeAgain with GLP-1 app to track vial ages. Building on the baseline you read earlier, I’ll show which technical tests and container choices actually determine that usable life, and what those choices mean for dosing reliability.
Beyond the Needle: Managing Your Body's Response to GLP-1s
Compounded semaglutide refers to custom-prepared formulations of semaglutide created by compounding pharmacies. When compounding teams select ingredients, every small choice affects the finished product's tolerance.
Different salt forms, buffers, preservatives, and even the source of the bulk API alter how the peptide responds to:
Heat
Oxygen
Time
Analytical labs measure those differences with methods like:
High-performance liquid chromatography and mass spectrometry for potency
Peptide mapping for chemical integrity
Validated sterility and endotoxin assays for microbiological safety
Those data justify a beyond-use date rather than a manufacturer's expiration date.
Shelf Life of Semaglutide Compound: Storage and Stability
Assigning a safe fridge life requires hard testing, not assumptions. Some facilities run real-time stability studies that monitor potency and degradation products over weeks under refrigerated conditions, while others rely on accelerated stability testing that extrapolates likely behavior. Reporting from STAT News, found that compounded semaglutide has a shelf life of 30 to 60 days, which explains why many compounding pharmacies set conservative BUDs when they lack extensive in-house stability data.
Given these short windows, staying organized with the MeAgain with GLP-1 app ensures you never lose track of a vial's potency. That range reflects uneven testing and variable container-closure systems, not a single chemical truth.
Factors That Affect Compounded Semaglutide Expiration
Certain variables predict shorter, less reliable fridge life:
Absorption into plastic
Preservative absence in multi-dose containers
Repeated needle punctures
Temperature excursions
The potency of compounded semaglutide can vary by up to 20%, according to STAT News, indicating that batch-to-batch variability is large enough to affect clinical response and complicate titration plans.
Freezing can irreversibly aggregate the peptide; agitation can expose it to oxygen and accelerate oxidation; and rubber stoppers with reactive extractables can subtly shift pH, all of which reduce useful potency even while a vial still appears normal.
Beyond the Sticky Note: Solving the Complexity of Long-Term GLP-1 Therapy
Most people handle BUDs with sticky notes and memory because that is simple and familiar. That works early on, but as refill batches and vial switches accumulate, missed expirations and inconsistent potency cause:
Missed doses
Confusing side effects
Wasted medication
Solutions like MeAgain centralize:
Batch numbers
BUDs
Temperature logs and automated reminders
It enables users and prescribers to spot a bad lot or an approaching expiration before a dose is drawn.
503B vs. 503A: Understanding the Gold Standard of Safety
You can reduce uncertainty by requesting evidence and by choosing practices that directly lower risk. Request a certificate of analysis demonstrating the assay methodology and potency for the lot you receive.
Ask whether the dispenser is a 503B outsourcing facility that performs validated stability and sterility testing, and prefer amber glass vials with intact, tested seals over unvalidated plastic containers.
Why Peptide Stability Can’t Be Eyeballed
Remember, refrigeration slows the chemical kinetics that drive deamidation and oxidation, like slowing a clock, but it does not pause those reactions; even small, repeated warmings or freeze-thaw cycles will accelerate breakdown.
If you want clinical confidence, insist on objective evidence:
Potency assay results
Sterility testing logs
Temperature-controlled shipping records
Digital solutions like the MeAgain with GLP-1 app centralize:
Batch numbers
BUDs
Temperature logs
It automates reminders so users can spot an approaching expiration before drawing a dose.
Bridging the Distance Between Lab Data and Dosing
If the pharmacy can demonstrate real-time stability and container-closure integrity for a specific formulation, clinicians can dose with predictable expectations; if not, conservative BUDs and single-dose formats are the safer path.
That gap between what you hope is in the vial and what lab data actually show is where real trouble starts, and it is far more common than people realize.
Related Reading
Semaglutide Weight Loss Side Effects
Who Should Not Take Semaglutide
Semaglutide Eye Side Effects
Compounded Semaglutide Side Effects
Semaglutide Belly Fat
Semaglutide Visceral Fat
Liraglutide vs Semaglutide
How to Store Compounded Semaglutide Safely and When to Stop Using It
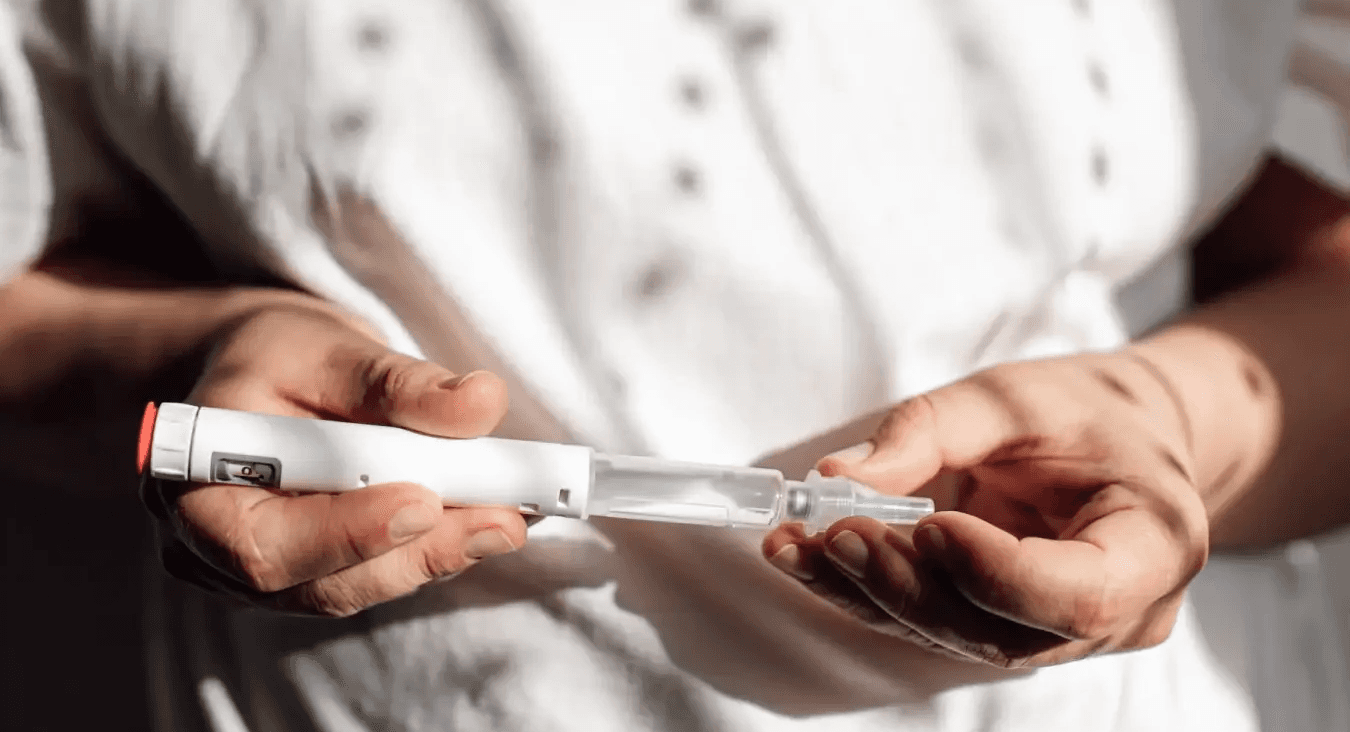
Follow the BUD: Never use a vial past the pharmacy-assigned beyond-use date.
Refrigerate correctly: Store at 2–8°C (36–46°F), in the main compartment, not the door or near freezer elements.
Protect from light: Keep in original amber or opaque container; wrap clear vials if needed.
Avoid contamination: Don’t transfer vials; clean rubber stoppers with alcohol before injections.
Travel smart: Use insulated coolers, avoid placing ice packs directly in contact with food, and carry on your flight.
Log every action: Track opened date, BUD, temperature exposures, and lot numbers.
Watch for signs of degradation: Discard if cloudy, colored, crystallized, or viscous, or if injection sites show redness, swelling, or pain.
Call the pharmacy when unsure: Provide lot number, BUD, storage details, and visual observations for guidance.
Don’t guess to save medication: Using compromised vials risks subpotent dosing, side effects, or infection.
Follow the beyond-use date printed on your compounding pharmacy's label, and do not use a vial after that date or after any visual change. If you notice cloudiness, particles, an unusual color, or signs of an injection-site infection, stop and call the pharmacy or your prescriber rather than guessing.
Optimizing Refrigerator Placement for Stability
Proper storage of compounded semaglutide is essential to maintain medication potency and safety throughout its beyond-use date. Store unopened and opened vials continuously in the refrigerator at 2°C to 8°C (36°F to 46°F). Place the medication in the main refrigerator compartment rather than the door, where temperature fluctuations occur with frequent opening.
Avoid storing near the freezer compartment or cooling elements, where accidental freezing can occur; frozen semaglutide should never be used, even after thawing, because freezing irreversibly damages the peptide structure.
Photodegradation: Protecting the Molecular Integrity of Your Dose
Protect compounded semaglutide from light exposure by keeping it in its original container, which should be amber or opaque. If your pharmacy provides clear vials, store them in the original box or wrap them in aluminum foil.
Never transfer compounded semaglutide to different containers, because that compromises sterility and makes tracking the beyond-use date difficult. Keep the medication away from direct sunlight and fluorescent lighting.
Maintaining the Cold Chain: Navigating Travel and Transit
When traveling with compounded semaglutide, use an insulated medication cooler with ice packs or gel packs to maintain the appropriate temperature. Ensure ice packs do not contact the vial directly, as this could cause localized freezing.
For flights, carry medication in carry-on luggage rather than checked baggage, where cargo hold temperatures may fall below freezing. The Transportation Security Administration permits medically necessary liquids exceeding 3.4 ounces when properly declared at security checkpoints.
Aseptic Discipline: Protecting Your Health from the First Puncture
Maintain a medication log noting the date you first puncture a multi-dose vial, and calculate the discard date per your pharmacy's instructions.
Use a permanent marker to write the “opened date” and “discard after” date directly on the vial label.
Clean the rubber stopper with alcohol before each injection to minimize contamination risk, following CDC injection safety guidelines.
Never share vials between patients, including family members, as this violates sterility protocols and poses a risk of infection transmission.
If you miss doses and approach the beyond-use date with significant medication remaining, consult your healthcare provider about whether to continue or obtain a fresh vial, and do not extend use beyond the assigned BUD.
Household Logistics: Turning Your Fridge into a Clinical Storage Unit
Pharmacies typically align their handling guidance with refrigerated temperature ranges; for example, “Refrigerator temperature should be between 36°F and 46°F / 2°C and 8°C”, Gobymeds, which means your household fridge settings and placement matter for chemical stability and consistent dosing.
“Compounded semaglutide has a Beyond-Use Date (BUD) of up to 60 days,” according to the same article, which explains why you must treat the pharmacy-assigned discard date as authoritative and plan refills around that window to avoid running out or using degraded product.
Signs Your Compounded Semaglutide May Be Expired
Recognizing signs of degraded or expired compounded semaglutide is crucial for medication safety and therapeutic effectiveness. The most obvious indicator is exceeding the beyond-use date printed on the vial label by your compounding pharmacy.
Using medication past this date is not recommended because potency cannot be guaranteed, and degradation products may have formed. Even if the solution appears normal, chemical changes invisible to the naked eye may have occurred.
Visual Bio-Integrity: How to Spot “The Unraveling” of a Peptide
Visual inspection provides important clues about medication integrity. Compounded semaglutide should appear as a clear, colorless to slightly yellow solution.
Discard the medication immediately if you observe any of the following:
Particulate matter
Color changes such as darkening or browning
Crystallization
Changes in viscosity
These physical changes indicate chemical degradation or contamination.
Beyond Potency: The Safety Hazards of “Visible Abnormalities”
The presence of visible particles is particularly concerning because this may:
Represent peptide aggregation
Interaction between the drug and container components
Even small particles that settle at the bottom of the vial warrant discarding the medication. Do not attempt to filter, heat, cool, or otherwise manipulate medication with any visible abnormalities. Cloudiness or turbidity suggests protein aggregation or bacterial growth.
Vigilant Monitoring: Distinguishing “Normal” Reactions from Infection
After injection, monitor for signs of possible contamination, including:
Fever
Increasing redness or warmth at the injection site
Swelling
Unusual pain
These symptoms could indicate infection and require immediate medical attention. While rare, contaminated injectable medications can cause serious infections.
Potency vs. Plateau: Identifying the Cause of Diminished Results
Reduced therapeutic effectiveness may indicate degraded medication, though this is difficult to assess without laboratory testing.
If you notice diminished appetite suppression, lack of expected weight loss, or rising blood glucose despite adherence, discuss with your healthcare provider whether medication potency may be compromised, as clinical factors can mimic degradation.
Beyond the Vial: Your Roadmap for Compromised Medication
If you suspect your compounded semaglutide has expired, been improperly stored, or shows signs of degradation, do not use it. Contact your compounding pharmacy and healthcare provider immediately.
Report adverse effects potentially related to degraded medication to the FDA MedWatch program. Proper disposal of expired or compromised medication is essential; return it to your pharmacy for safe disposal rather than discarding it in household trash or flushing it down the drain.
What Should I Tell The Pharmacy When I Call?
Start with clear facts, not assumptions: give the vial lot number, the beyond-use date on the label, when you first opened it, and the precise timing of any room-temperature exposures. Say whether you observed visual changes and whether you stored them in the main compartment or the door.
Ask for the pharmacy’s documented guidance for that lot and whether they will replace doses if the BUD is reached early due to a confirmed storage failure. This level of detail allows the pharmacist to assess sterility risk and replacement policy, rather than relying on vague reports.
How Do I Document And Escalate If Needed?
Keep a quick record:
Photo of the label showing lot and BUD
Timestamped photos of any visual abnormality
A short note on temperatures or travel events
If the pharmacy recommends discarding, request a written note or email you can share with your prescriber to authorize a refill. If you suspect contamination caused an adverse reaction, seek medical care and request that the clinic document the episode and retain the vial for pharmacy or public health review.
Why Not Just Guess and Save The Vial?
Most people try to stretch doses when cost or access feels tight, because it is familiar and simple. That familiar approach becomes costly when potency declines or infection risk increases; a subpotent dose can delay expected benefits and lead to unnecessary dose escalations, and an infection can result in urgent care visits.
In short, the small habit of “making it last” creates outsized clinical and financial friction over the course of weeks.
How Can Tools Make Tracking Reliable?
Platforms like MeAgain provide a different option: they:
Centralize BUDs
Batch numbers
Temperature logs
This is why people stop relying on sticky notes and memory. Users find that automatic reminders, photo storage for label images, and temperature and event logging reduce accidental use of expired products and make refill timing predictable, lowering anxiety and preventing wasted medication.
What To Do If You See Injection-Site Signs Of Infection?
Seek urgent medical evaluation if you:
Have a fever
Spreading redness
Worsening pain at the injection site
Keep the vial and packaging sealed and take photos to share with clinicians and the dispensing pharmacy. Clinical teams may request cultures or advise empiric treatment; the vial can be important evidence if microbial contamination is suspected.
Think of vial handling like a passport for each dose: dates, origin, and a simple temperature trail preserve trust between you, the pharmacist, and the prescriber. That simple recordkeeping feels small until missing it costs you weeks of progress, and that’s where things get complicated.
Related Reading
• How to Track My Injections on Semaglutide?
• How Much Protein Should I Eat on Semaglutide
• Best App for Tracking Semaglutide Results
• What to Do When Semaglutide Stops Working
• How to Inject Semaglutide
• Semaglutide Foods to Avoid
• Semaglutide Body Composition
• How to Track Food on Semaglutide
• Semaglutide for Weight Loss in Non-Diabetics Dosage
• How Can I Track My Semaglutide Progress?
• How to Track My Semaglutide Side Effects?
Download MeAgain to Stay on Track With Your GLP-1 Medication and Protect Your Results Without Stress or Wasted Doses
I know starting Ozempic, Wegovy, or Mounjaro can feel overwhelming when dosing schedules, side effects, and medication timelines are involved.
MeAgain helps you stay consistent and confident by turning the daily work of GLP-1 treatment into a simple, motivating game. Our friendly capybara keeps you focused on the habits that support safe, effective weight loss so you’re not guessing or falling behind, like hitting your:
Protein
Fiber
Hydration
Movement goals
You can also capture your progress with the Journey Card, helping you remember milestones as your body changes faster than expected.
MeAgain is the all-in-one GLP-1 companion that makes staying healthy easier, clearer, and more motivating, not stressful. Download MeAgain and turn your weight loss journey into your favorite game.

